Nadolol
(Preusmjereno sa stranice VWPOSFSPZNDTMJ-UCWKZMIHSA-N)
Nadolol (Corgard, Anabet, Solgol, Corzide, Alti-Nadolol, Apo-Nadol, Novo-Nadolol) je neselektivni beta blokator koji se koristi za lečenje visokog krvnog pritiska, migrenskih glavobolja, i bola u grudima.[6][7]
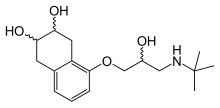
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| rel-(2R,3S)-5[(2R)-3-(tert-butilamino)-2-hidroksipropil]oksi1,2,3,4-tetrahidronafthalen-2,3-diol (2R*,3S*)-5[(2R*)-3-(tert-butilamino)-2-hidroksipropil]oksi1,2,3,4-tetrahidronafthalen-2,3-diol | |||
| Klinički podaci | |||
| Robne marke | Corgard | ||
| AHFS/Drugs.com | Monografija | ||
| MedlinePlus | a682666 | ||
| Identifikatori | |||
| CAS broj | 42200-33-9 | ||
| ATC kod | C07AA12 | ||
| PubChem[1][2] | 39147 | ||
| DrugBank | DB01203 | ||
| ChemSpider[3] | 35815 | ||
| UNII | FEN504330V | ||
| KEGG[4] | D00432 | ||
| ChEMBL[5] | CHEMBL649 | ||
| Hemijski podaci | |||
| Formula | C17H27NO4 | ||
| Mol. masa | 309,401 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Vezivanje za proteine plazme | 30% | ||
| Metabolizam | Nil | ||
| Poluvreme eliminacije | 14-24 sata | ||
| Izlučivanje | Renalno i fekalno (nepromenjen) | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | C(US) | ||
| Pravni status | POM (UK) ℞-only (SAD) | ||
| Način primene | Oralnot | ||
Povezano
urediReference
uredi- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Hardman JG, Limbird LE, Gilman AG. (2001). Goodman & Gilman's The Pharmacological Basis of Therapeutics (10 izd.). New York: McGraw-Hill. DOI:10.1036/0071422803. ISBN 0-07-135469-7.
- ↑ Pdr Staff (2009). PDR: Physicians Desk Reference 2010 (Physicians' Desk Reference (Pdr)). Rozelle, N.S.W: Thomson Reuters. ISBN 1-56363-748-0.
Literatura
uredi- Buice RG, Subramanian VS, Duchin KL, Uko-Nne S. (1996). „Bioequivalence of a highly variable drug: an experience with nadolol”. Pharmaceutical Research 13 (7): 1109–15. DOI:10.1023/A:1016031313065. PMID 8842054.
- N. Hanania, S. Singh, R. El-Wali, et al. The safety and effects of the beta-blocker, nadolol, in mild asthma: An open-label pilot study, Pulmonary Pharmacology & Therapeutics, Volume 21, Issue 1, Pages 134-141
Vanjske veze
uredi- Nadolol Arhivirano 2007-08-22 na Wayback Machine-u