N-Metilserotonin
N-Metilserotonin je triptaminski alkaloid. Hemijski, on je derivat serotonina u kome se metil grupa nalazi na alkil aminu. On se takođe naziva Nω-metilserotonin (Nω-metil-5-hidroksitriptamin).
| N-Metilserotonin | |||
|---|---|---|---|
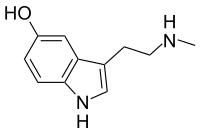
| |||
| IUPAC ime |
| ||
| Drugi nazivi | Norbufotenin; Nω-Metilserotonin; N-Metil-5-hidroksitriptamin | ||
| Identifikacija | |||
| CAS registarski broj | 1134-01-6 | ||
| PubChem[1][2] | 150885 | ||
| ChemSpider[3] | 132989 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C11H14N2O | ||
| Molarna masa | 190.24 g mol−1 | ||
|
| |||
| Infobox references | |||
N-Metilserotonin je prisutan u biljkama, životinjama, i gljivama. To obuhvata biljke, Actaea racemosa[4] i Zanthoxylum piperitum,[5] žabe Litoria aurea,[6] i Amanita pečurke.[7] Ovo jedinjenje se vezuje za nekoliko serotoninskih receptora, uključujući 5-HT7 i 5-HT1A receptore, sa visokim afinitetom (IC50 ≤ 2 nM) i selektivnošću.
Reference uredi
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Powell, SL; Gödecke, T; Nikolic, D; Chen, SN; Ahn, S; Dietz, B; Farnsworth, NR; Van Breemen, RB i dr.. (2008). „In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent”. Journal of Agricultural and Food Chemistry 56 (24): 11718–26. DOI:10.1021/jf803298z. PMID 19049296.
- ↑ Yanase, E; Ohno, M; Harakawa, H; Nakatsuka, SI (2010). „Isolation of N,N-dimethyl and N-methylserotonin 5-O-β-glucosides from immature Zanthoxylum piperitum seeds”. Bioscience, Biotechnology, and Biochemistry 74 (9): 1951–2. DOI:10.1271/bbb.100261. PMID 20834148.
- ↑ McClean, Stephen; Robinson, Robert C.; Shaw, Chris; Smyth, W. Franklin (2002). „Characterization and determination of indole alkaloids in frog-skin secretions by electrospray ionization ion trap mass spectrometry”. Rapid Communications in Mass Spectrometry 16 (5): 346–354. DOI:10.1002/rcm.583. PMID 11857717.
- ↑ Tyler, V. E., Jr.; Groeger, D. (1964). „Amanita alkaloids. II. Amanita citrina and Amanita porphyria”. Planta Medica 12 (4): 397–402. DOI:10.1055/s-0028-1100193.