Ciprofloksacin (INN) pripada drugoj generaciji fluorohinolonskih antibiotika.[7][8] Njegov spektar delovanja obuhvata većinu sojeva bakterijskih patogena odgovornih za respiratorne, urinarne, gastrointestinalne, i abdomenalne infekcije, uključujući Gram-negativne (Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Legionella pneumophila, Moraxella catarrhalis, Proteus mirabilis, i Pseudomonas aeruginosa), i Gram-positivne (meticilin-senzitivni ali ne meticilin-rezistentni Staphylococcus aureus, Streptococcus pneumoniae, Staphylococcus epidermidis, Enterococcus faecalis, i Streptococcus pyogenes) bakterijske patogene. Ciprofloksacin i drugi fluorohinoloni su vredni zbog njihovog širokog spektra aktivnosti, odlične penetracije tkiva, kao i njihove dostupnosti u oralnim i intravenskim formulacijama.[9]
Ciprofloksacin
|
|
| (IUPAC) ime
|
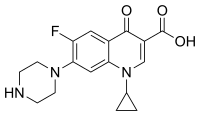
| 1-ciklopropil-6-fluoro-4-okso-7-(piperazin-1-il)-hinolin-3-karboksilna kiselina
|
| Klinički podaci
|
| Robne marke
|
Ciloxan, Cipro, Neofloxin
|
| AHFS/Drugs.com
|
Monografija
|
| MedlinePlus
|
a688016
|
| Identifikatori
|
| CAS broj
|
85721-33-1
|
| ATC kod
|
J01MA02 S01AE03 S02AA15 S03AA07
|
| PubChem[1][2]
|
2764
|
| DrugBank
|
DB00537
|
| ChemSpider[3]
|
2662
|
| UNII
|
5E8K9I0O4U  Y Y
|
| KEGG[4]
|
D00186  Y Y
|
| ChEBI
|
CHEBI:100241  Y Y
|
| ChEMBL[5]
|
CHEMBL8  Y Y
|
| Hemijski podaci
|
| Formula
|
C17H18FN3O3
|
| Mol. masa
|
331,346
|
| SMILES
|
eMolekuli & PubHem
|
| InChI |
|---|
InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24)  Y Y
Key: MYSWGUAQZAJSOK-UHFFFAOYSA-N  Y Y |
|
| Farmakokinetički podaci
|
| Bioraspoloživost
|
69%[6]
|
| Metabolizam
|
Hepatički, uključujući CYP1A2
|
| Poluvreme eliminacije
|
4 sata
|
| Izlučivanje
|
Renal
|
| Farmakoinformacioni podaci
|
| Licenca
|
US FDA:link
|
| Trudnoća
|
B3(AU) C(US)
|
| Pravni status
|
Samo na recept (S4) (AU) POM (UK) ℞-only (SAD)
|
| Način primene
|
Oralno, intravenozno, topikalno
|
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594. edit
- ↑ Drusano GL, Standiford HC, Plaisance K, Forrest A, Leslie J, Caldwell J (September 1986). „Absolute oral bioavailability of ciprofloxacin”. Antimicrob Agents Chemother. 30 (3): 444–6. ISSN 0066-4804. PMC 180577. PMID 3777908.
- ↑ Nelson, JM.; Chiller, TM.; Powers, JH.; Angulo, FJ. (Apr 2007). „Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story.”. Clin Infect Dis 44 (7): 977–80. DOI:10.1086/512369. PMID 17342653.
- ↑ Kawahara, S. (Dec 1998). „[Chemotherapeutic agents under study]”. Nippon Rinsho 56 (12): 3096–9. PMID 9883617.
- ↑ Brunton, Laurence; Lazo, John; Parker, Keith (2005). Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill Prof Med/Tech. ISBN 978-0-07-142280-2. Pristupljeno 30. 10. 2012.

