Kodein
Kodein ili 3-metilmorfin (prirodni izomer od metil morfina) je opijat koji se koristi u medicini kao analgetik i antidiuretik.[6] Kodein je drugi alkaloid po dominantnosti u opijumu (do 3 odsto); mnogo više preovladava u iranskom maku (Papaver bractreatum). Smatra se drogom srednje klase.
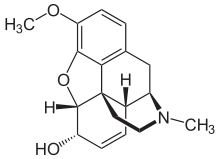
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| (5α,6α)-7,8-didehydro-4,5-epoxy-3-methoxy-17-methylmorphinan-6-ol | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 76-57-3 | ||
| ATC kod | R05DA04 N02AA59 | ||
| PubChem[1][2] | 5284371 | ||
| DrugBank | APRD00120 | ||
| ChemSpider[3] | 4447447 | ||
| ChEMBL[4] | CHEMBL485 | ||
| Hemijski podaci | |||
| Formula | C18H21NO3 | ||
| Mol. masa | 299.364 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Bioraspoloživost | ~90% Oralno | ||
| Metabolizam | Hepatički, putem CYP2D6 (citohroma P450 2D6)[5] | ||
| Poluvreme eliminacije | 2.5–3 h | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | Droga (S8) (AU) Schedule I (CA) ? (UK) Schedule II (SAD) | ||
| Opojna droga | nisko - umereno | ||
| Način primene | oralno, intra-rektalno, supkutano, intramaskularno | ||
Kodein se koristi za lečenje bolova, kao i za oslobađanje od kašlja.
Izvori uredi
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Shen H, He MM, Liu H, et al (August 2007). „Comparative metabolic capabilities and inhibitory profiles of CYP2D6.1, CYP2D6.10, and CYP2D6.17”. Drug Metab. Dispos. 35 (8): 1292–300. DOI:10.1124/dmd.107.015354. PMID 17470523.
- ↑ Keith Parker; Laurence Brunton; Goodman, Louis Sanford; Lazo, John S.; Gilman, Alfred (2006). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11 izd.). New York: McGraw-Hill. ISBN 0-07-142280-3.