Pirometamin je jedan od antagonista folne kiseline koji se koristi kao antimalarijalni agens ili sa sulfonamidom za tretma toksoplazmoze.[6][7][8][9]
Pirimetamin
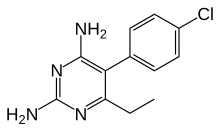
|
| (IUPAC) ime
|
| 5-(4-hlorofenil)-6-etilpirimidin-2,4-diamin
|
| Klinički podaci
|
| Robne marke
|
Darachlor, Daraclor, Darapram, Daraprim
|
| AHFS/Drugs.com
|
Monografija
|
| Identifikatori
|
| CAS broj
|
58-14-0
|
| ATC kod
|
P01BD01
|
| PubChem[1][2]
|
4993
|
| DrugBank
|
DB00205
|
| ChemSpider[3]
|
4819
|
| KEGG[4]
|
C07391  Y Y
|
| ChEMBL[5]
|
CHEMBL8673  Y Y
|
| Hemijski podaci
|
| Formula
|
C12H13ClN4
|
| Mol. masa
|
248.711
|
| SMILES
|
eMolekuli & PubHem
|
| InChI |
|---|
InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17)
Key: WKSAUQYGYAYLPV-UHFFFAOYSA-N  Y Y |
|
| Fizički podaci
|
| Tačka topljenja
|
233.5 °C (452 °F)
|
| Farmakokinetički podaci
|
| Poluvreme eliminacije
|
96 sata
|
| Farmakoinformacioni podaci
|
| Trudnoća
|
?
|
| Pravni status
|
|
| Način primene
|
Oralno
|
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594. edit
- ↑ Gatton ML, Martin LB, Cheng Q: Evolution of resistance to sulfadoxine-pyrimethamine in Plasmodium falciparum. Antimicrob Agents Chemother. 2004 Jun;48(6):2116-23. PMID 15155209
- ↑ Sirichaiwat C, Intaraudom C, Kamchonwongpaisan S, Vanichtanankul J, Thebtaranonth Y, Yuthavong Y: Target guided synthesis of 5-benzyl-2,4-diamonopyrimidines: their antimalarial activities and binding affinities to wild type and mutant dihydrofolate reductases from Plasmodium falciparum. J Med Chem. 2004 Jan 15;47(2):345-54. PMID 14711307
- ↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035-41. PMID 21059682.
- ↑ Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic Acids Res 36 (Database issue): D901-6. PMID 18048412.
Spoljašnje veze
uredi
