Fluor perhlorat
(Preusmjereno sa stranice KUHLYXODBIVLJW-UHFFFAOYSA-N)
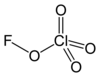
Fluor perhlorat je hemijsko jedinjenje, koje ima molekulsku masu od 122,481 Da.
| Fluor perhlorat | |||
|---|---|---|---|
 |
 | ||
| Identifikacija | |||
| CAS registarski broj | 10049-03-3 | ||
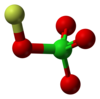
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | H4ClFO4 | ||
| Molarna masa | 122.48 g mol−1 | ||
|
| |||
| Infobox references | |||
Osobine
uredi| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 4 |
| Broj donora vodonika | 3 |
| Broj rotacionih veza | 1 |
| Particioni koeficijent[1] (ALogP) | 0,3 |
| Rastvorljivost[2] (logS, log(mol/L)) | -0,3 |
| Polarna površina[3] (PSA, Å2) | 69,9 |
Reference
uredi- ↑ Ghose, A.K., Viswanadhan V.N., and Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A 102: 3762-3772. DOI:10.1021/jp980230o.
- ↑ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE. (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488-1493. DOI:10.1021/ci000392t. PMID 11749573.
- ↑ Ertl P., Rohde B., Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714-3717. DOI:10.1021/jm000942e. PMID 11020286.
Literatura
uredi- Holleman A. F., Wiberg E. (2001). Inorganic Chemistry (1st edition izd.). San Diego: Academic Press. ISBN 0-12-352651-5.
- Housecroft C. E., Sharpe A. G. (2008). Inorganic Chemistry (3rd izd.). Prentice Hall. ISBN 978-0-13-175553-6.