Guvacin
Guvacin je piridinski alkaloid prisutan u Areka orahu. On je eksperimentalni lek, bez odobrene indikacije. Eksperimentalne studije se sprovode da bi se utvrdila njegova fiziološka svojstva i mehanizmi dejstva. Poznato je da je guvacin specifičan inhibitor GABA preuzimanja bez znatnog afiniteta za GABAB receptore.[4][5][6]
| Guvacin | |||
|---|---|---|---|
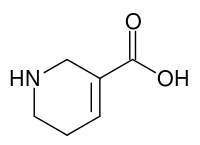
| |||
| Identifikacija | |||
| CAS registarski broj | 498-96-4 | ||
| PubChem[1][2] | 3532 | ||
| DrugBank | DB08848 | ||
| KEGG[3] | |||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C6H9NO2 | ||
| Molarna masa | 127.14 g mol−1 | ||
| Tačka topljenja |
306 | ||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
Osobine
uredi| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 3 |
| Broj donora vodonika | 2 |
| Broj rotacionih veza | 1 |
| Particioni koeficijent[7] (ALogP) | -2,7 |
| Rastvorljivost[8] (logS, log(mol/L)) | -0,6 |
| Polarna površina[9] (PSA, Å2) | 49,3 |
Reference
uredi- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Krogsgaard-Larsen P, Frolund B, Frydenvang K: GABA uptake inhibitors. Design, molecular pharmacology and therapeutic aspects. Curr Pharm Des. 2000 Aug;6(12):1193-209. PMID 10903390
- ↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035-41. DOI:10.1093/nar/gkq1126. PMC 3013709. PMID 21059682.
- ↑ David S. Wishart, Craig Knox, An Chi Guo, Dean Cheng, Savita Shrivastava, Dan Tzur, Bijaya Gautam, and Murtaza Hassanali (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic Acids Res 36 (Database issue): D901-6. DOI:10.1093/nar/gkm958. PMC 2238889. PMID 18048412.
- ↑ Ghose, A.K., Viswanadhan V.N., and Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A 102: 3762-3772. DOI:10.1021/jp980230o.
- ↑ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE. (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488-1493. DOI:10.1021/ci000392t. PMID 11749573.
- ↑ Ertl P., Rohde B., Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714-3717. DOI:10.1021/jm000942e. PMID 11020286.