1,2-Naftohinon
1,2-Naftohinon (orto-naftohinon) je policiklično aromatično organsko jedinjenje.
| 1,2-Naftohinon | |||
|---|---|---|---|
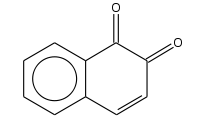
| |||
| Drugi nazivi | o-Naftohinon, β-naftohinon | ||
| Identifikacija | |||
| CAS registarski broj | 524-42-5 | ||
| PubChem[1][2] | 10667 | ||
| ChemSpider[3] | 10217 | ||
| KEGG[4] | |||
| ChEMBL[5] | CHEMBL52347 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C10H6O2 | ||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
Ovaj dvostruki keton (hinon) je reaktivni metabolit naftalina, koji je prisutan je u česticama dizelnih izduvnih gasova. Akumulacija ovog toksičnog metabolita izaziva oštećenje očiju kod pacova, uključujući formiranje katarakti.[6]
Reference
uredi- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Qian, W.; Shichi, H. (2001). „Naphthoquinone-Induced Cataract in Mice: Possible Involvement of Ca2+ Release and Calpain Activation”. Journal of Ocular Pharmacology and Therapeutics 17 (4): 383–392. DOI:10.1089/108076801753162799. PMID 11572469.
Literatura
uredi- Troester, M. A.; Lindstrom, A. B.; Waidyanatha, S.; Kupper, L. L.; Rappaport, S. M. (2002). „Stability of Hemoglobin and Albumin Adducts of Naphthalene Oxide, 1,2-Naphthoquinone, and 1,4-Naphthoquinone” (pdf). Toxicological Sciences 68 (2): 314–321. DOI:10.1093/toxsci/68.2.314. PMID 12151627.
- Kikuno, S.; Taguchi, K.; Iwamoto, N. et al. (2006). „1,2-Naphthoquinone Activates Vanilloid Receptor 1 through Increased Protein Tyrosine Phosphorylation, Leading to Contraction of Guinea Pig Trachea”. Toxicology and Applied Pharmacology 210 (1–2): 47–54. DOI:10.1016/j.taap.2005.06.015. PMID 16039679.
Povezano
uredi- 1,4-Naftohinon, izomer 1,2-naftohinona