Tandospiron
Tandospiron (Sediel, metanopiron) je anksiolitik i antidepresiv koji se koristi u Kini i Japanu. On je član azapironske i piperazinske hemijske klase, i blisko je srodan sa drugim agensima poput buspirona i gepirona.
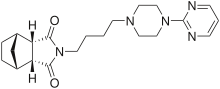
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| (1R,2R,6S,7S)-44-[4-(pirimidin-2-il)piperazin-1-il]butil4-azatriciklo[5.2.1.02,6]dekan-3,5-dion | |||
| Klinički podaci | |||
| AHFS/Drugs.com | Internacionalno ime leka | ||
| Identifikatori | |||
| CAS broj | 112457-95-1 | ||
| ATC kod | nije dodeljen | ||
| PubChem[1][2] | 91273 | ||
| ChemSpider[3] | 82421 | ||
| UNII | 190230I669 | ||
| ChEMBL[4] | CHEMBL274047 | ||
| Hemijski podaci | |||
| Formula | C21H29N5O2 | ||
| Mol. masa | 383,487 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Poluvreme eliminacije | 1,2–1,4 sata | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | ℞ Prescription only | ||
| Način primene | Oralno | ||
Farmakologija uredi
Tandospiron deluje kao potentan i selektivan parcijalni agonist 5-HT1A receptora, sa Ki afinitetom od 27 ± 5 nM[5] i aproksimativno 55-85% intrinsične aktivnosti.[6][7] On ima slab i klinički zanemarljiv afinitet za 5-HT2A (1,300 ± 200), 5-HT2C (2,600 ± 60), α1-adrenergički (1,600 ± 80), α2-adrenergički (1,900 ± 400), D1 (41,000 ± 10,000), i D2 (1,700 ± 300) receptore, i esencijalno je neaktivan na 5-HT1B, 5-HT1D, β-adrenergičkom, i muskarinskim acetilholinskim receptorima, serotoniskom transportertu (SERT), i benzodiazepinskom (BDZ) alosternom mestu GABAA receptora (svi od kojih su > 100.000).[5] Postoji evidencija da tandospiron ima nisku, mada značajnu antagonističku aktivnost na α2-adrenergičkom receptoru putem njegovog aktivnog metabolita 1-(2-pirimidinil)piperazin (1-PP).[8][9]
Hemija uredi
Tandospiron se može sintetisati na sledeće način:[10]
Reference uredi
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ 5,0 5,1 Hamik; Oksenberg, D; Fischette, C; Peroutka, SJ (1990). „Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites”. Biological Psychiatry 28 (2): 99–109. DOI:10.1016/0006-3223(90)90627-E. PMID 1974152.
- ↑ Tanaka; Tatsuno, T; Shimizu, H; Hirose, A; Kumasaka, Y; Nakamura, M (1995). „Effects of tandospirone on second messenger systems and neurotransmitter release in the rat brain”. General pharmacology 26 (8): 1765–72. DOI:10.1016/0306-3623(95)00077-1. PMID 8745167.
- ↑ Yabuuchi, Kazuki; Tagashira, Rie; Ohno, Yukihiro (2004). „Effects of tandospirone, a novel anxiolytic agent, on human 5-HT1A receptors expressed in Chinese hamster ovary cells (CHO cells)”. Biogenic Amines 18 (3): 319. DOI:10.1163/1569391041501933.
- ↑ Blier; Curet, O; Chaput, Y; De Montigny, C (1991). „Tandospirone and its metabolite, 1-(2-pyrimidinyl)-piperazine--II. Effects of acute administration of 1-PP and long-term administration of tandospirone on noradrenergic neurotransmission”. Neuropharmacology 30 (7): 691–701. DOI:10.1016/0028-3908(91)90176-C. PMID 1681447.
- ↑ Miller; Thompson, ML; Byrnes, JJ; Greenblatt, DJ; Shemer, A (1992). „Kinetics, brain uptake, and receptor binding of tandospirone and its metabolite 1-(2-pyrimidinyl)-piperazine”. Journal of Clinical Psychopharmacology 12 (5): 341–5. PMID 1362206.
- ↑ Yevich, Joseph P.; New, James S.; Smith, David W.; Lobeck, Walter G.; Catt, John D.; Minielli, Joseph L.; Eison, Michael S.; Taylor, Duncan P. i dr.. (1986). „Synthesis and biological evaluation of 1-(1,2-benzisothiazol-3-yl)- and (1,2-benzisoxazol-3-yl)piperazine derivatives as potential antipsychotic agents”. Journal of Medicinal Chemistry 29 (3): 359–69. DOI:10.1021/jm00153a010. PMID 2869146.