Protopin
Protopin je benzilizohinolinski alkaloid koji se javlja u maku[5], Corydalis krtolama[6] i drugim biljkama iz familije papaveraceae, kao i Fumaria officinalis.[7] Utvrđeno je da inhibira histaminske H1 receptore i zgrušavanje krvi, i da deluje kao analgetik.[8]
| Protopin[1] | |||
|---|---|---|---|
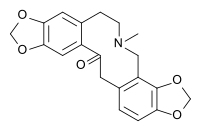
| |||
| IUPAC ime |
| ||
| Identifikacija | |||
| CAS registarski broj | 130-86-9 | ||
| PubChem[2][3] | 4970 | ||
| ChEMBL[4] | CHEMBL486179 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| Svojstva | |||
| Molekulska formula | C20H19NO5 | ||
| Molarna masa | 353,369 g/mol | ||
| Agregatno stanje | beli kristali | ||
| Gustina | 1,399 g/cm3 | ||
| Tačka topljenja |
208 °C | ||
| Rastvorljivost u vodi | nerastvoran je | ||
| Rastvorljivost u hloroform | 1:15 | ||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
Reference uredi
- ↑ The Merck Index (9 izd.). New Jersey: Merck & Co. 1976. str. 1023.
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ The Free Dictionary: Protopine
- ↑ Jiang, B; Cao, K; Wang, R (2004). „Inhibitory effect of protopine on K(ATP) channel subunits expressed in HEK-293 cells.”. European Journal of Pharmacology 506 (2): 93-100. DOI:10.1016/j.ejphar.2004.11.004. PMID 15588728.
- ↑ Vrba, J.; Vrublova, E.; Modriansky, M.; Ulrichova, J. (2011). „Protopine and allocryptopine increase mRNA levels of cytochromes P450 1A in human hepatocytes and HepG2 cells independently of AhR”. Toxicology Letters 203 (2): 135-141. DOI:10.1016/j.toxlet.2011.03.015. PMID 21419197.
- ↑ Saeed, SA; Gilani, AH; Majoo, RU; Shah, BH (1997). „Anti-thrombotic and anti-inflammatory activities of protopine.”. Pharmacological research : the official journal of the Italian Pharmacological Society 36 (1): 1-7. DOI:10.1006/phrs.1997.0195. PMID 9368908.