Dehidroepiandrosteron
Dehidroepiandrosteron (DHEA, Fidelin, androstenolon, prasteron, 3β-hidroksiandrost-5-en-17-on, 5-androsten-3β-ol-17-on) je važan endogeni steroidni hormon.[5] On je najzastupljeniji circulišući steroid kod ljudi,[6] kod kojih ga proizvode nadbubrežne žlezde,[7] gonade, i mozak,[8] gde on predominentno funkcioniše kao metabolički intermedijer u biosintezi androgena i estrogena seksualnih steroida.[9][5] Međutim, DHEA takođe ima raznovrsne samostalne potencijalne biološke efekte. On se vezuje za niz nuklearnih površinskih receptora,[10] i deluje kao neurosteroid.[11]

| |||
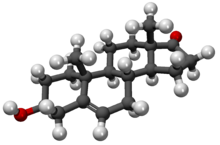
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| (3S,8R,9S,10R,13S,14S)-3-hidroksi-10,13-dimetil-1,2,3,4,7,8,9,11,12,14,15,16-dodekahidrociklopenta[a]fenantren-17-on | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 53-43-0 | ||
| ATC kod | A14AA07 G03EA03 (kombinacija sa estrogenom) | ||
| PubChem[1][2] | 5881 | ||
| DrugBank | DB01708 | ||
| ChemSpider[3] | 5670 | ||
| UNII | 459AG36T1B | ||
| ChEBI | CHEBI:28689 | ||
| ChEMBL[4] | CHEMBL90593 | ||
| Hemijski podaci | |||
| Formula | C19H28O2 | ||
| Mol. masa | 288,424 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Sinonimi | (3β)-3-Hidroksiandrost-5-en-17-on | ||
| Fizički podaci | |||
| Tačka topljenja | 148.5 °C (299 °F) | ||
| Farmakokinetički podaci | |||
| Metabolizam | Hepatički | ||
| Poluvreme eliminacije | 12 sata | ||
| Izlučivanje | Urinarno:?% | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | Komercijalno dostupan | ||
| Način primene | Oralno | ||
Istraživanja uredi
Kancer uredi
Više in vitro studija je utvrdilo da DHEA ima antiproliferativne i apoptozne efekte na ćelijama kancera.[12][13][14] Kliničkih značaj tih nalaza nije poznat. Visoki nivoi DHEA i drughi endogenih seks hormona su u znatnoj meri vezani za povišeni rizik od razvoja raka dojki pre- i postmenopozalno.[15][16]
Reference uredi
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ 5,0 5,1 Mo Q, Lu SF, Simon NG (April 2006). „Dehydroepiandrosterone and its metabolites: differential effects on androgen receptor trafficking and transcriptional activity”. J. Steroid Biochem. Mol. Biol. 99 (1): 50–8. DOI:10.1016/j.jsbmb.2005.11.011. PMID 16524719.
- ↑ William F Ganong MD, 'Review of Medical Physiology', 22nd Ed, McGraw Hill, 2005, page 362.
- ↑ The Merck Index, 13th Edition, 7798
- ↑ Schulman, Robert A., M.D.; Dean, Carolyn, M.D. (2007). Solve It With Supplements. New York City: Rodale, Inc.. str. 100. ISBN 978-1-57954-942-8. »DHEA (Dehydroepiandrosterone) is a common hormone produced in the adrenal glands, the gonads, and the brain.«
- ↑ Thomas Scott (1996). Concise Encyclopedia Biology. Walter de Gruyter. str. 49. ISBN 978-3-11-010661-9. Pristupljeno 25. 5. 2012.
- ↑ Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK (2006). „The biological actions of dehydroepiandrosterone involves multiple receptors”. Drug Metabolism Reviews 38 (1–2): 89–116. DOI:10.1080/03602530600569877. PMC 2423429. PMID 16684650.
- ↑ Friess E, Schiffelholz T, Steckler T, Steiger A (December 2000). „Dehydroepiandrosterone--a neurosteroid”. European Journal of Clinical Investigation 30 Suppl 3: 46–50. DOI:10.1046/j.1365-2362.2000.0300s3046.x. PMID 11281367.
- ↑ Yang, N. C.; Jeng, K. C.; Ho, W. M.; Hu, M. L. (2002). „ATP depletion is an important factor in DHEA-induced growth inhibition and apoptosis in BV-2 cells”. Life Sci. 70 (17): 1979–88. DOI:10.1016/S0024-3205(01)01542-9. PMID 12148690.
- ↑ Schulz, S.; Klann, R. C.; Schönfeld, S.; Nyce, J. W. (1992). „Mechanisms of cell growth inhibition and cell cycle arrest in human colonic adenocarcinoma cells by dehydroepiandrosterone: role of isoprenoid biosynthesis”. Cancer Res. 52 (5): 1372–6. PMID 1531325.
- ↑ Loria, R. M. (2002). „Immune up-regulation and tumor apoptosis by androstene steroids”. Steroids 67 (12): 953–66. DOI:10.1016/S0039-128X(02)00043-0. PMID 12398992.
- ↑ Tworoger, S. S.; Missmer, S. A.; Eliassen, A. H. et al. (2006). „The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women”. Cancer Epidemiol. Biomarkers Prev. 15 (5): 967–71. DOI:10.1158/1055-9965.EPI-05-0976. PMID 16702378.
- ↑ Key, T.; Appleby, P.; Barnes, I.; Reeves, G. (2002). „Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies”. J. Natl. Cancer Inst. 94 (8): 606–16. DOI:10.1093/jnci/94.8.606. PMID 11959894.
Literatura uredi
- Thomas Scott (1996). Concise Encyclopedia Biology. Walter de Gruyter. str. 49. ISBN 978-3-11-010661-9. Pristupljeno 25. 5. 2012.
- Schulman, Robert A., M.D.; Dean, Carolyn, M.D. (2007). Solve It With Supplements. New York City: Rodale, Inc.. str. 100. ISBN 978-1-57954-942-8. »DHEA (Dehydroepiandrosterone) is a common hormone produced in the adrenal glands, the gonads, and the brain.«
Spoljašnje veze uredi
- Information on DHEA from the Mayo Clinic
- DHEA in elderly women and DHEA or testosterone in elderly men, published in the New England Journal of Medicine in 2006. "Neither DHEA nor low-dose testosterone replacement in elderly people has physiologically relevant beneficial effects on body composition, physical performance, insulin sensitivity, or quality of life."
- DHEA, from the Skeptic's Dictionary
- ChemSub Online: Dehydroepiandrosterone - DHEA