Pilokarpin
Pilokarpin je parasimpatomimetički alkaloid koji se dobija iz lišća tropskih američkih žbunova iz roda Pilocarpus. On je neselektivni agonist muskarinskog receptora[7] u parasimpatičkom nervnom sistemu, sa terapeutskim dejstvom na muskarinski acetilholinski receptor M3. On se primenjuje topički,[8] npr., u lečenju glaukoma i kserostomije.
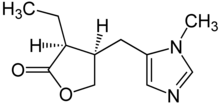
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| (3S,4R)- 3-etil- 4-((1-metil- 1H-imidazol- 5-il) metil)dihidrofuran- 2(3H)-on | |||
| Klinički podaci | |||
| Robne marke | Salagen | ||
| AHFS/Drugs.com | Monografija | ||
| MedlinePlus | a608039 | ||
| Identifikatori | |||
| CAS broj | 92-13-7 54-71-7 (hidrohlorid) | ||
| ATC kod | N07AX01 S01EB01 | ||
| PubChem[1][2] | 5910 | ||
| DrugBank | APRD00382 | ||
| ChemSpider[3] | 5699 | ||
| UNII | 01MI4Q9DI3 | ||
| KEGG[4] | D00525 | ||
| ChEBI | CHEBI:8207 | ||
| ChEMBL[5] | CHEMBL550 | ||
| Hemijski podaci | |||
| Formula | C11H16N2O2 | ||
| Mol. masa | 208.257 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Poluvreme eliminacije | 0,76 sata (5 mg), 1,35 sata (10 mg)[6] | ||
| Izlučivanje | urin | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | ℞-only (SAD) | ||
| Način primene | kapi, oralno | ||
Reference uredi
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Mervyn Gornitsky, George Shenouda, Khalil Sultanem, Howard Katz, Michael Hier, Martin Black, Ana M Velly (2004). „Double-blind randomized, placebo-controlled study of pilocarpine to salvage salivary gland function during radiotherapy of patients with head and neck cancer”. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 98 (1): 45-52.
- ↑ Spalding et al. 2002.
- ↑ Pharmacology, (Rang, Dale, Ritter & Moore, ISBN 0-443-07145-4, 5:th ed., Churchill Livingstone 2003) Page 144.
Literatura uredi
- The Italian Journal of Neurological Sciences Volume 16, Numbers 1-2, 33-37, DOI: 10.1007/BF02229072
- Pathophysiology of Status Epilepticus Induced by Pilocarpine, central nervous system agents in medical chemistry, 2007, vol. 7, no. 1