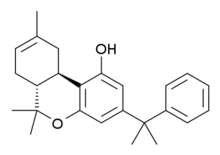
KM-233 je leki koji je analogan sa Δ8-tetrahidrokanabinolom (THC), manje potentnim ali stablinijim izomerom aktivne komponente kanabisa. KM-233 se razlikuje od Δ8-THC po tome što je pentilni bočni lanac zamenjen 1,1-dimetilbenzil grupom. On ima visok in vitro afinitet vezivanja za CB1 i CB2 receptore, sa CB2 afinitetom od 0,91 nM i 13x selektivnošću u odnosu na CB1 receptor.[3] U životinjskim studijama je utvrđeno da je efektivan u tretmanu glioma, forme moždanog tumora.[4] Veliki broj srodnih analoga je poznat, kod kojih je 1,1-dimetilbenzil grupa supstituisana drugim grupama.[5][6][7][8][9]
KM-233
|
| (IUPAC) ime
|
| (−)-(6aR,7,10,10aR)-tetrahidro-6,6,9-trimetil-3-(1-metil-1-feniletil)-6H-dibenzo[b,d]piran-1-ol
|
| Klinički podaci
|
| Identifikatori
|
| CAS broj
|
628263-22-9
|
| ATC kod
|
nije dodeljen
|
| PubChem[1][2]
|
10248110
|
| Hemijski podaci
|
| Formula
|
C25H30O2
|
| Mol. masa
|
362,503 g/mol
|
| SMILES
|
eMolekuli & PubHem
|
| InChI |
|---|
InChI=1S/C25H30O2/c1-16-11-12-20-19(13-16)23-21(26)14-18(15-22(23)27-25(20,4)5)24(2,3)17-9-7-6-8-10-17/h6-11,14-15,19-20,26H,12-13H2,1-5H3  Y Y
Key: GZYXCXRHVALIJD-UHFFFAOYSA-N  Y Y |
|
| Farmakoinformacioni podaci
|
| Trudnoća
|
?
|
| Pravni status
|
?
|
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Krishnamurthy M, Ferreira AM, Moore BM 2nd. Synthesis and testing of novel phenyl substituted side-chain analogues of classical cannabinoids. Bioorganic and Medicinal Chemistry Letters. 2003 Oct 20;13(20):3487-90. PMID 14505654
- ↑ Duntsch C, et al. Safety and efficacy of a novel cannabinoid chemotherapeutic, KM-233, for the treatment of high-grade glioma. Journal of Neuro-oncology. 2006 Apr;77(2):143-52. PMID 16314952
- ↑ Nadipuram AK, et al. Synthesis and testing of novel classical cannabinoids: exploring the side chain ligand binding pocket of the CB1 and CB2 receptors. Bioorganic and Medicinal Chemistry. 2003 Jul 17;11(14):3121-32. PMID 12818675
- ↑ Durdagi S, et al. The application of 3D-QSAR studies for novel cannabinoid ligands substituted at the C1' position of the alkyl side chain on the structural requirements for binding to cannabinoid receptors CB1 and CB2. Journal of Medicinal Chemistry. 2007 Jun 14;50(12):2875-85. PMID 17521177
- ↑ Krishnamurthy M, Gurley S, Moore BM 2nd. Exploring the substituent effects on a novel series of C1'-dimethyl-aryl Delta8-tetrahydrocannabinol analogs. Bioorganic and Medicinal Chemistry. 2008 Jul 1;16(13):6489-500. PMID 18524604
- ↑ Ferreira AM, et al. Quantitative structure-activity relationship (QSAR) for a series of novel cannabinoid derivatives using descriptors derived from semi-empirical quantum-chemical calculations. Bioorganic and Medicinal Chemistry. 2009 Mar 15;17(6):2598-606. PMID 19250829
- ↑ Brogi S, et al. Three-dimensional quantitative structure-selectivity relationships analysis guided rational design of a highly selective ligand for the cannabinoid receptor 2. European Journal of Medicinal Chemistry. 2011 Feb;46(2):547-55. PMID 21183257
Spoljašnje veze
uredi
