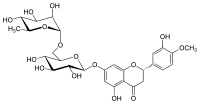
Hesperidin
Hesperidin je flavanonski glikozid koji je izobilan u citrusnom voću. Njegova aglikonska forma se naziva hesperetin. Ime potiče od hesperidnih nimfi iz Grčke mitologije. Za hesperidin se misli da učestvuje u odbrambenom mehanizmu biljki. Sudeći po in vitro ispitivanjima, on deluje kao antioksidans.[5]
| Hesperidin | |||
|---|---|---|---|
| |||
| IUPAC ime |
| ||
| Identifikacija | |||
| CAS registarski broj | 520-26-3 | ||
| PubChem[1][2] | 10621 | ||
| ChemSpider[3] | 10176 | ||
| UNII | E750O06Y6O | ||
| ChEBI | 28775 | ||
| ChEMBL[4] | CHEMBL449317 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C28H34O15 | ||
| Molarna masa | 610.56 g mol−1 | ||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
Razna preliminarna ispitivanja indiciraju da on ima specifične farmaceutske osobine, mada nije potvrđeno da su one primenljive kod ljudi. Hesperidin redukuje novo holesterola[6] i krvnog pritiska[7] kod pacova. U jednoj studiji na miševima, velike doze hesperidina su snizile gubitak gustine kostiju.[8] Jedna druga studija na životinjama je pokazala protektivne efekte protiv sepse.[9] U in vitro i laboratorijskim istraživanjima, hesperidin pokazuje antiinflamatorno dejstvo.[10][11] Hesperidin je isto tako potencijalni sedativ. Moguće je da on deluje putem opioidnih ili adenozinskih receptora.[12][13] Hesperidin pokazuje izraženo antikancerno dejstvo protiv nekih ljudskih karcinomnih ćelijskih linija.[14]
Deo in vitro rezultata je primenljiv samo na aglikonsku formu. Hesperidin takođe ima sposobnost penetracije krvno moždane barijere u in vitro modelima.[15]
Reference uredi
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S (2005). „Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression”. Anticancer Res. 25 (5): 3367–74. PMID 16101151.
- ↑ Monforte MT, Trovato A, Kirjavainen S, Forestieri AM, Galati EM, Lo Curto RB (September 1995). „Biological effects of hesperidin, a Citrus flavonoid. (note II): hypolipidemic activity on experimental hypercholesterolemia in rat”. Farmaco 50 (9): 595–9. PMID 7495469.
- ↑ Ohtsuki K, Abe A, Mitsuzumi H, et al. (December 2003). „Glucosyl hesperidin improves serum cholesterol composition and inhibits hypertrophy in vasculature”. J. Nutr. Sci. Vitaminol. 49 (6): 447–50. PMID 14974738.
- ↑ Chiba H, Uehara M, Wu J, et al. (June 2003). „Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice”. J. Nutr. 133 (6): 1892–7. PMID 12771335.
- ↑ Kawaguchi K, Kikuchi S, Hasunuma R, Maruyama H, Yoshikawa T, Kumazawa Y (May 2004). „A citrus flavonoid hesperidin suppresses infection-induced endotoxin shock in mice”. Biol. Pharm. Bull. 27 (5): 679–83. DOI:10.1248/bpb.27.679. PMID 15133244.
- ↑ Emim JA, Oliveira AB, Lapa AJ (February 1994). „Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice”. J. Pharm. Pharmacol. 46 (2): 118–22. PMID 8021799.
- ↑ Galati EM, Monforte MT, Kirjavainen S, Forestieri AM, Trovato A, Tripodo MM (November 1994). „Biological effects of hesperidin, a citrus flavonoid. (Note I): antiinflammatory and analgesic activity”. Farmaco 40 (11): 709–12. PMID 7832973.
- ↑ Loscalzo LM, Wasowski C, Paladini AC, Marder M (February 2008). „Opioid receptors are involved in the sedative and antinociceptive effects of hesperidin as well as in its potentiation with benzodiazepines”. Eur. J. Pharmacol. 580 (3): 306–13. DOI:10.1016/j.ejphar.2007.11.011. PMID 18048026.
- ↑ Guzmán-Gutiérrez SL, Navarrete A (March 2009). „Pharmacological exploration of the sedative mechanism of hesperidin identified as the active principle of Citrus sinensis flowers”. Planta Med. 75 (4): 295–301. DOI:10.1055/s-0029-1185306. PMID 19219759.
- ↑ Al-Ashaal HA, El-Sheltawy ST"Antioxidant capacity of hesperidin from citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm Biol. 2011 Mar;49(3):276-82
- ↑ Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C (April 2003). „Interaction between flavonoids and the blood-brain barrier: in vitro studies”. J. Neurochem. 85 (1): 180–92. DOI:10.1046/j.1471-4159.2003.01652.x. PMID 12641740.