Droperidol
Droperidol (Inapsine, Droleptan, Dridol, Xomolix, Innovar [kombinacija sa fentanilom]) je antidopaminergički lek koji se koristi kao antiemetik i antipsihotik.[5][6] Droperidol se takođe koristi za neuroleptanalgezijsku anesteziju i sedaciju pri intenzivnoj nezi.
| |||
| |||
| (IUPAC) ime | |||
|---|---|---|---|
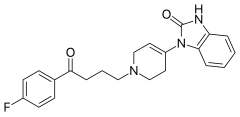
| 1-{1-[4-(4-fluorofenil)-4-oksobutil]-1,2,5,6-tetrahidropiridin-4-il}-1,3-dihidro-2H-benzimidazol-2-on | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 548-73-2 | ||
| ATC kod | N05AD08 | ||
| PubChem[1][2] | 3168 | ||
| DrugBank | DB00450 | ||
| UNII | O9U0F09D5X | ||
| KEGG[3] | D00308 | ||
| ChEMBL[4] | CHEMBL1108 | ||
| Hemijski podaci | |||
| Formula | C22H22FN3O2 | ||
| Mol. masa | 379,428 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Metabolizam | Hepatički | ||
| Poluvreme eliminacije | 2,3 sata | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | ℞-only (SAD) | ||
| Način primene | Intravenozno, Intramaskularno | ||
Hemija uredi
Droperidol se može sintetisati iz 1-benzil-3-karbetoksipiperidin-4-ona, reakcijom sa o-fenilendiaminom.[7][8][9][10]
Reference uredi
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035-41. PMID 21059682.
- ↑ Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic Acids Res 36 (Database issue): D901-6. PMID 18048412.
- ↑ C. Janssen, NV Res. Lab., GB 989755 (1962).
- ↑ Janssen, P. A. J.; 1963, Belgian Patent BE 626307.
- ↑ F.J. Gardocki, J. Janssen, U.S. Patent 3.141.823 (1964).
- ↑ P.A.J. Janssen, U.S. Patent 3.161.645 (1964).
Literatura uredi
- Scuderi PE: Droperidol: Many questions, few answers. Anesthesiology 2003; 98: 289-90
- Lischke V, Behne M, Doelken P, Schledt U, Probst S, Vettermann J. Droperidol causes a dose-dependent prolongation of the QT interval. Department of Anesthesiology and Resuscitation, Johann Wolfgang Goethe-University Clinics, Frankfurt am Main, Germany.
- Emergency Medicine Magazine : http://www.emedmag.com/html/pre/tri/1005.asp Arhivirano 2011-05-27 na Wayback Machine-u