Karmustin
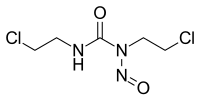
Karmustin ili BCNU (bis-hloroetilnitrozoureja) je β-hloro-nitrozourejno jedinjenje strodno sa azotnim iperitom, koje se koristi kao alkilirajući agens u hemoterapiji.[7][8] Kao dialkilirajući agens, BCNU ima sposobnost formiranja veza između DNK lanaca, čime se onemogućava DNK replikacija i DNK transkripcija.
| Karmustin | |||
|---|---|---|---|
| |||

| |||
| IUPAC ime |
| ||
| Drugi nazivi | N,N’-Bis(2-hloroetil)-N-nitrozo-ureja | ||
| Identifikacija | |||
| CAS registarski broj | 154-93-8 | ||
| PubChem[2][3] | 2578 | ||
| ChemSpider[4] | 2480 | ||
| UNII | U68WG3173Y | ||
| EINECS broj | |||
| UN broj | 2811 | ||
| DrugBank | DB00262 | ||
| KEGG[5] | |||
| MeSH | |||
| ChEBI | 3423 | ||
| ChEMBL[6] | CHEMBL513 | ||
| RTECS registarski broj toksičnosti | YS2625000 | ||
| ATC code | L01 | ||
| Jmol-3D slike | |||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C5H9Cl2N3O2 | ||
| Molarna masa | 214.05 g mol−1 | ||
| Agregatno stanje | Orange crystals-lat | ||
| Miris | Bez mirisa | ||
| Tačka topljenja |
30 °C, 303 K, 86 °F | ||
| log P | 1,375 | ||
| pKa | 10,194 | ||
| Baznost (pKb) | 3,803 | ||
| Farmakologija | |||
| Bioraspoloživost | 5–28% | ||
| Načini upotrebe | Intravenozno | ||
| Metabolizam | Hepatički | ||
| Vreme polu-uklanjanja iz organizma | 15–30 min | ||
| Proteinsko vezivanje | 80% | ||
| Ekskrecija |
| ||
| Legalni status |
| ||
| Opasnost | |||
| EU-klasifikacija | |||
| R-oznake | R45, R46, R60, R61, R28 | ||
| S-oznake | S22, S36/37/39, S45 | ||
| LD50 | 20 mg kg−1 (oralno, pacov) | ||
| Srodna jedinjenja | |||
| Srodna ureje | Dimetilureja | ||
| Srodna jedinjenja | |||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
It has the appearance of an orange-yellow solid.
Carmustine for injection is marketed under the name BiCNU by Bristol-Myers Squibb. In India, carmustine is marketed under the name Carustine by Curacell Biotech.
Reference uredi
- ↑ „Carmustine - Compound Summary”. PubChem Compound. USA: National Center for Biotechnology Information. 25. 3. 2005.. Identification. Pristupljeno 11. 4. 2012.
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035-41. PMID 21059682.
- ↑ Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic Acids Res 36 (Database issue): D901-6. PMID 18048412.
Spoljašnje veze uredi
- MedlinePlus DrugInfo medmaster-a682060
- BiCNU Arhivirano 2007-09-27 na Wayback Machine-u (package insert; U.S.)