2-Amino-1-metil-6-fenilimidazo(4,5-b)piridin
PhIP (2-Amino-1-metil-6-fenilimidazo(4,5-b)piridin) jedan je od najzastupljenijih heterocikličnigh amina (HCA) u kuvanom mesu. PhIP se formira na visokim temperaturama reakcijom između kreatina ili kreatinina (prisutnog u mišićnom tkivu), aminokiselina, i šećera. PhIP formiranje se povećava sa povećanjem temperature i dužinom kuvanja, a takođe je zavisno od metoda kuvanja i tipa mesa. Smatra se da može da bude kancirogen.[5][6].[7]
| 2-Amino-1-metil-6-fenilimidazo(4,5-b)piridin | |||
|---|---|---|---|
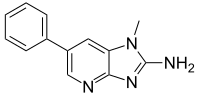
| |||
| IUPAC ime |
| ||
| Drugi nazivi | PhIP | ||
| Identifikacija | |||
| CAS registarski broj | 105650-23-5 | ||
| PubChem[1][2] | 1530 | ||
| ChemSpider[3] | 1476 | ||
| DrugBank | DB08398 | ||
| ChEMBL[4] | CHEMBL1213271 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C13H12N4 | ||
| Molarna masa | 224.26 g mol−1 | ||
| Agregatno stanje | bela čvrsta materija | ||
| Gustina | 1,3 gcm−3 | ||
| Tačka topljenja |
300 °C, 573 K, 572 °F | ||
| Tačka ključanja |
468.9 °C, 742 K, 876 °F | ||
| Rastvorljivost u vodi | 407,1 mg/L | ||
| Opasnost | |||
| Opasnost u toku rada | T | ||
|
| |||
| Infobox references | |||
Reference uredi
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program. (2011). Report on Carcinogens, 12th ed., p. 222.
- ↑ International Agency for Research on Cancer (IARC) (1997) PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine)(Group 2B). Summaries & Evaluations. http://www.inchem.org/documents/iarc/vol56/08-phip.html
- ↑ Cross, A., & Sinha, R. (2004) Meat-Related Mutagens/Carcinogens in the Etiology of Colorectal Cancer. Environmental and Molecular Mutagenesis. 44:45-55.